In vitro Antidiabetic Studies of Aqueous Extract of Pleurotus ostreatus Grown on Different Substrates
| Received 21 Mar, 2023 |
Accepted 30 Jul, 2023 |
Published 26 Aug, 2023 |
Background and Objective: Nature-derived products are finding increased application for therapeutic purposes. Apart from their use as food, mushrooms have been reported in various research to contain bioactive compounds with medicinal capabilities. This study evaluated the in vitro antidiabetic and antioxidant activities of an aqueous extract of Pleurotus ostreatus (mushroom) cultivated on rice bran/sawdust (RB/SD, 70:30 w/w) mixture and sawdust (SD) substrates. Materials and Methods: Pleurotus ostreatus was cultivated on two different substrates followed by aqueous extraction of mature mushrooms. In vitro antidiabetic, (α-amylase and α-glucosidase inhibition) and antioxidant (DPPH radical-scavenging assay and ferric reducing power (FRAP) activities were done using standard methods. Results were analyzed using IBM Statistical Product and Service Solutions (SPSS) software, version 23 and the significance level was established at p<0.05. Results: The extracts showed a concentration-dependent inhibitory effect on α-amylase and α-glucosidase activities in vitro. There was a stronger inhibition of α-amylase activity by the RB/SD sample when compared to SD and a weaker inhibitory effect of the RB/SD sample on α-glucosidase activity when compared to SD. Again, the samples showed a concentration-dependent antioxidant effect with increased DPPH-scavenging activity and FRAP observed with the SD sample compared to the RB/SD sample. The GC-FID analysis of the P. ostreatus samples also revealed the presence of a number of bioactive compounds in different concentrations. Conclusion: These results highlight the effect of cultivation substrate on the potential therapeutic effect of mushrooms the aqueous extract of P. ostreatuscould be a promising raw material for the development of anti-diabetes therapeutics.
INTRODUCTION
Diabetes mellitus (DM), one of the leading causes of morbidity and mortality globally, is a multifactorial chronic and complicated metabolic disorder characterized by elevated serum glucose levels caused by the body’s inability to produce, recognize or respond to insulin and thus leads to a variety of complications1,2. The American Diabetes Association (ADA) and the International Diabetes Federation (IDF) reported that about 2.2% of the world’s population dies yearly as a result of DM3 and estimated a 50% increase in people living with diabetes by 2045 with the number of diabetic people reaching 629 million4. Diabetes itself is seemingly harmless, however, prolonged and untreated presentations lead to various complications such as retinopathy, neuropathy and diabetic foot amongst others. Diabetic complications arise due to persistent hyperglycemia and hyperglycemia-induced oxidative stress5. Thus, effective glycemic control appears as a viable option in the management of diabetes and associated complications.
Inhibition of the glucose-releasing enzymes α-amylase and α-glucosidase is one of the therapeutic options presently explored for effective glycemic control. The α-amylase plays an important role in starch digestion by catalyzing the hydrolysis of α-(1, 4)-D-glycosidic linkages in starch, producing smaller fragments and other polymers of glucose6,7. Its products are further degraded by α-glucosidase to absorbable monosaccharides which then enters the bloodstream8,9. Inhibiting these enzymes would, therefore, hinder starch absorption, expand intestinal sugar holding time and delay the rate of glucose absorption into the bloodstream. Approved therapeutic drugs that target these enzymes include acarbose, voglibose and migiltol. These, however, cause unwanted side effects such as diarrhea, bloating, flatulence and abdominal discomfort thus limiting their use in diabetes therapy10,11.
The use of natural remedies for disease management is a common practice by mankind from the beginning of time. Advancements in technology has also made it possible to identify and study the safety and therapeutic profiles of the many bioactive components of many natural products12. Mushrooms are highly effective nutraceutical products, conferring many health benefits in addition to their nutritional properties13-15. Hepatoprotective properties of species such as Auricularia auricular, Tricholoma lobayense, Grifola frondosa, Tremella fuciformis, Flammulina velutipes, Lentinula edodes and Volvariella volvacea have been reported and attributed to a variety of inherent molecular entities in these mushrooms16. Chaga mushroom (Inonotus obliquus) has also been recognized for its potential to be used against the SARS-CoV2 virus17. Various reports also suggest that mushrooms have the ability to maintain normoglycemia with little or no side effects2,3,11,18. Pleurotus ostreatus, a member of the genus Pleurotus has attracted a lot of attention due to various advantages over other species due to its capacity to thrive at a variety of temperatures19,20. As a rich source of nutrients and other bioactive compounds, P. ostreatus exhibits a wide range of therapeutic functions including immuno-modulatory, antiviral, anti-oxidative, cardioprotective and antitumor12,21,22. Studies have shown that the nutritional and bioactive component of mushrooms depend greatly on substrate used in mushroom cultivation as mushrooms are potent bio-accumulators23-26. Hence, this study evaluates the in vitro antidiabetic and antioxidant effect of the aqueous extract of Pleurotus ostreatus grown on rice bran/sawdust combination and sawdust alone.
MATERIALS AND METHODS
Study duration: The study was carried out between December, 2021 and April 2022, lasting for a period of four months.
Materials
Collection and authentication of materials: The materials used for this study include Pleurotus ostreatus, rice bran and wood sawdust. The mushroom (Pleurotus ostreatus) used was cultivated on a mixture of rice bran (1.75 kg) and sawdust (0.75 kg) (70:30 w/w) combination and sawdust alone substrate (2.5 kg) at the South East Zonal Biotechnology Center, University of Nigeria, Nsukka. Wood sawdust was purchased in Timbre market Adani and rice bran was purchased in Timbre Market Nsukka both in Uzo-Uwani LGA area, Enugu State. Both were milled to fine particles.
Chemicals and reagents: All chemicals and reagents used in this study were of analytical grade. They were obtained from reputable chemical dealers in Nsukka, Enugu State and Onitsha, Anambra State, both in Nigeria. They include Potassium sodium tartrate (Rochette salt) (HD, China DNSA (Sigma, USA), Acetic acid and Sodium acetate (HD, China). Starch extract was gotten from Xanthosoma sagittafolium, while Sucrose was a product of lobachemie. The enzymes alpha-amylase and alpha-glucosidase was purchased from the Megazyme manufacturing company.
Methods
Spawn preparation: The mushroom spawn was prepared as described by Thongklang and Luangharn27. Pleurotus ostreatus mycelium was cultured on potato dextrose agar (PDA) followed by inoculation of the spawn on parboiled sorghum seeds. This was maintained as the stock culture for mushroom cultivation.
Preparation of substrate and cultivation of mushroom: Substrates used for mushroom cultivation, rice-bran/sawdust 70:30 w/w combination and sawdust only, were prepared as described by Chukwurah et al.28 and Aguchem et al.29. Cultivation of mushrooms was also carried out as described by Aguchem et al.29.
Preparation of mushroom extract: As 35.87 g of fresh mushroom sample was weighed using an analytical balance (Thermo Fisher, USA). It was put in an electric blender (Binatone, Hong Kong, China) containing 60 mL of distilled water and was blended for 5 min. It was then filtered using a filter cloth to obtain the filtrate. The filtrate was centrifuged at 1000×g for 15 min and the supernatant was collected and stored in a small container as the aqueous extract.
Determination of inhibitory effect of the P. ostreatus aqueous extract on α-amylase and α-glucosidase activities in vitro: The procedure described by Narkhede et al.30 with few modifications was used in determining the inhibitory effect of the P. ostreatus aqueous extract on α-amylase and α-glucosidase activities in vitro. The assay mixture containing the solution of the enzymes, α-amylase and α-glucosidase, (0.1 mL), acetate buffer (0.1-0.5 mL, 0.1M at pH 5.5) and mushroom extracts (32-160 μg mL–1), respectively were added to five test tubes labelled test tube 1-5 and incubated for 15 min at room temperature. Afterwards, 0.5 mL of 2% sucrose and 2% starch was added to the test tubes as substrate for the enzymes and incubated in a controlled water bath at 50°C for 30 min. The reaction was terminated with the addition of 1 mL of dinitrosalicylic acid (DNSA) reagent and placed in a boiling water bath for 10 min. To stabilize the colour after heating, 1 mL of 1.4 M Rochelle salt (sodium potassium tartarate) was added to the test tubes immediately31 and the total volume of the solution was adjusted to 4 mL with distilled water. The reaction mixture was cooled to room temperature and absorbance was taken at 540 nm. The test tubes used as blank were prepared without mushroom extract.
Percentage inhibition of enzyme activity was calculated as described by Narkhede et al.30:
Where:
| A540 Ctrl | = | Absorbance of control at 540 nm | |
| A540 treatment | = | Absorbance of a sample containing extract at 540 nm |
In vitro antioxidant activity of the P. ostreatus aqueous extract
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay: The DPPH radical-scavenging activity of the extract was determined following the protocol of Sayah et al.32. Differently labelled test tubes received additions of the mushroom extracts at various amounts (ranging from 32 to 160 mg mL–1) followed by the addition of water to bring the volume to 250 L. Each test tube was then shaken violently following the addition of 2 mL of a 0.18 mM (0.005%) methanolic solution of DPPH after which the mixture was allowed to stand in the dark for 30 minutes at room temperature. The same procedure was used to prepare the control without any extract. Changes in samples absorbances were taken at 517 nm using a spectrophotometer (Spectron lab 2A, England). DPPH radical scavenging potential was calculated using the method provided by Adebiyi et al.33:
Ferric reducing antioxidant power assay: The method described by Alachaher et al.34 was used to determine the ferric reducing antioxidant potential of the mushroom extract. One milliliter of a solution containing 32, 64, 96, 128 and 160 mg mL–1 each of the extracts was combined with potassium ferricyanide (5.0 mL, 1.0%), sodium phosphate buffer (5.0 mL, 0.2 M) and potassium phosphate buffer (0.2 M, pH 6.6). The mixture was incubated at 50°C for 20 min followed by the addition of 5 mL of 10% trichloroacetic acid and centrifuged at 1000×g. The upper layer of the solution (5.0 mL) was diluted with 5.0 mL of distilled water and ferric chloride and absorbance was measured at 700 nm using a spectrophotometer (Spectron lab 2A, England). The experiment was performed thrice and results were averaged.
Extraction of chemical constituents: Extraction of chemical constituents was carried out following Ojukwu et al.35. As 15 mL ethanol and 10 mL 50% w/v KOH was added to a test tube containing one (1 g) of sample and the test tube was allowed to sit in a water bath at 60°C for 1 hr. The content was then transferred to a separating funnel and washed successively with ethanol (20 mL), cold water (10 mL), hot water (10 mL) and hexane (3 mL), which was all transferred to the funnel. The resultant solution was further washed three times with 10 mL of 10% v/v aqueous ethanol solution and dried with anhydrous sodium sulfate. The resulting sample was solubilized in 1000 μL of pyridine after which 200 μL (0.2 mL) of the solution was transferred to a vial for analysis.
Quantification of chemical constituents by gas chromatography-flame ionization detector: Determination of the chemical constituents was done on a flame ionization detector (FID)-equipped gas chromatography machine (BUCK M910, PUB Scientist, USA). As 0.1 mL of the extract was drawn using a syringe and injected into the gas chromatography (GC) machine equipped with FID. In principle, the detector uses a flame to ionize carbon-containing organic compounds. During separation, the sample passes through a hydrogen-fueled flame in the GC column which ionizes the carbon atoms in the sample.
Statistical analysis: Results were analyzed using IBM Statistical Product and Service Solutions (SPSS) software, version 23. They were analyzed using One-way Analysis of Variance (ANOVA) and presented as Mean±Standard Deviation (n = 3). The results were considered to have significant statistical differences when p<0.05. The IC50 values were calculated by linear regression analysis.
RESULTS
Inhibitory effect of the mushroom extracts on α-amylase and α-glucosidase activities in vitro: Table 1 shows the effect of the mushroom extracts on α-amylase and α-glucosidase activities in vitro. The result showed that both samples had a concentration-dependent inhibitory effect on both α-amylase (IC50 value of 745.22 and 1456.84 μg mL–1, respectively for SD and RB/SD) and α-glucosidase (IC50 value of I525.91 and 795.99 μg mL–1, respectively for SD and RB/SD) activities in vitro. From the result, the SD sample showed a stronger inhibitory effect on α-glucosidase activity than α-amylase while the RB/SD sample had a stronger inhibitory effect on α-amylase activity compared to α-glucosidase activity.
In vitro antioxidant activity of the mushroom samples: Table 2 shows the in vitro antioxidant activity of the aqueous homogenates of the mushroom samples. From the result, a concentration-related DPPH radical-scavenging activity was observed for both mushroom samples with IC50 values of 257.99 and 127.80 mg mL–1 for RB/SD and SD samples respectively. The samples also showed good ferric reducing power in a concentration-dependent manner with the SD sample showing stronger reducing power compared to the RB/SD sample. It was observed from the result that the SD sample had higher antioxidant activity in vitro compared to the RB/SD sample.
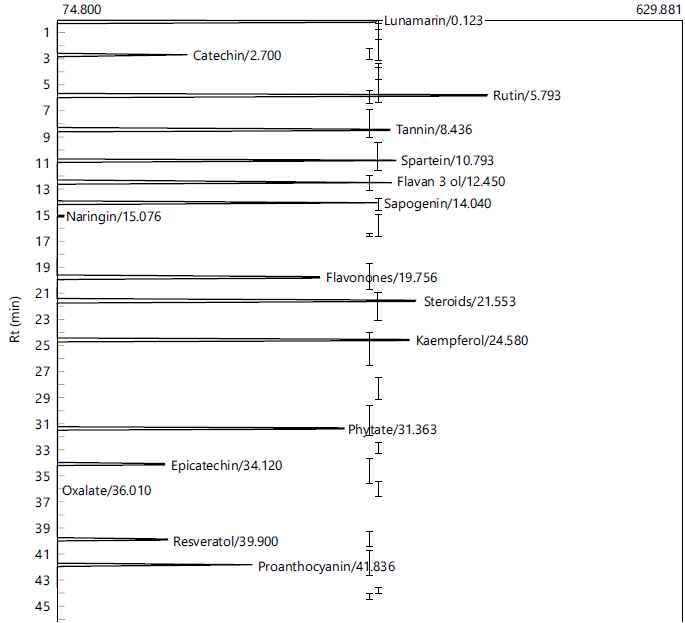
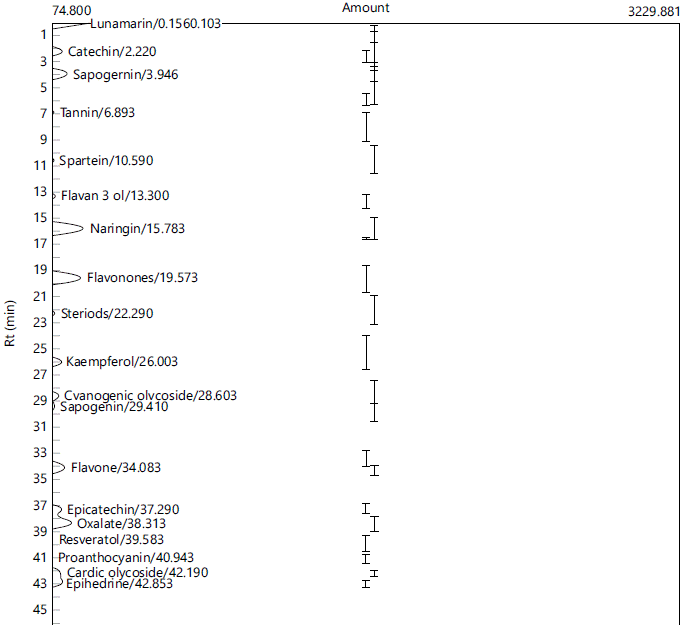
Chemical constituents of the extracts of P. ostreatus grown on rice bran/sawdust (70:30 w/w) combination (RB/SD) and sawdust (SD): The gas chromatography analysis of the mushroom samples (Fig. 1 and 2) revealed the presence of several chemical compounds including catechin, epicatechin, rutin, kaempferol, lunamarin, spartein, resveratrol, naringin in varying concentrations. Epicatechin, catechin, kaempferol, resveratrol and sapogenin were higher in the RB/SD sample compared to the SD sample, while rutin, spartein and lunamarin were higher in the SD sample compared to the RB/SD sample (Table 3).
| Table 1: | Effect of Pleurotus ostreatus aqueous extracts α-amylase and α-glucosidase activities in vitro | |||
α-amylase inhibition |
α-glucosidase inhibition |
|||
| Sample concentration (μg mL–1) |
SD (inhibition (%)) |
RB/SD (inhibition (%)) |
SD (inhibition (%)) |
RB/SD (inhibition (%)) |
| 32 | 10.39±0.36a |
16.84±0.48a |
15.17±0.65a |
5.89±2.36a |
| 64 | 10.71±0.47a |
19.87±0.49b |
16.43±0.39a |
8.01±0.63b |
| 96 | 10.86±0.41a |
19.84±0.14b |
16.84±0.42a |
12.19±1.69c |
| 128 | 12.89±0.61b |
19.94±0.24b |
20.09±0.49b |
12.27±0.43c |
| 160 | 18.50±0.61c |
20.40±0.54b |
24.99±0.46c |
12.85±0.58c |
IC50 = 745.22 μg mL–1 |
IC50 = 1456.84 μg mL–1 |
IC50 = I525.91 μg mL–1 |
IC50 = 795.99 μg mL–1 |
|
| Data represent Mean±Standard Deviation (n = 3), values with different superscripts down the column are significantly different at p<0.05, SD: Saw dust, RB: Rice bran and RB/SD: Rice bran/saw dust substrate combination |
||||
| Table 2: | In vitro antioxidant activity of the mushroom samples | |||
DPPH-scavenging activity |
FRAP |
|||
| Sample concentration (mg mL–1) | RB/SD (inhibition (%)) | SD (inhibition (%)) | RB/SD | SD |
| 32 | 23.43±0.20a | 28.63±0.63a | 8.01±0.21a | 68.20±0.55a |
| 64 | 25.39±0.41b | 38.70±3.98b | 50.04±0.13b | 73.33±0.27b |
| 96 | 26.83±1.01b | 38.27±2.03b | 49.81±0.21b | 79.18±1.27c |
| 128 | 35.85±0.26c | 50.38±5.92c | 55.10±0.40c | 82.83±0.12d |
| 160 | 39.85±1.80d | 58.50±1.44d | 58.07±0.32d | 85.47±3.25d |
| Data represent Mean±Standard Deviation (n = 3), values with different superscript in rows are significantly different at p<0.05, SD: Saw dust, RB: Rice bran and RB/SD: Rice bran/saw dust substrate combination | ||||
|
| Table 3: | Chemical constituents of the aqueous extract of P. ostreatus grown on rice bran/sawdust (70:30 w/w) combination (RB/SD) and sawdust (SD) | |||
| Component | RB/SD (μg mL–1) |
SD (μg mL–1) |
| Lunamarin | 2.714 |
8.486 |
| Catechin | 5.769 |
1.797 |
| Sapogenin | 8.233 |
ND |
| Rutin | ND |
3.611 |
| Tannin | 2.999 |
3.153 |
| Spartein | 1.821 |
2.015 |
| Flavan-3-ol | 2.868 |
2.81 |
| Naringin | 10.501 |
0.856 |
| Resveratrol | 2.381 |
1.255 |
| Flavonone | 7.414 |
2.326 |
| Steroids | 6.419 |
8.037 |
| Kaempferol | 6.055 |
4.46 |
| Cyanogenic glycoside | 5.786 |
ND |
| Phytate | ND |
5.504 |
| Flavone | 6.766 |
ND |
| Epicatechin | 35.198 |
12.692 |
| Oxalate | 8.512 |
1.554 |
| Cardiac glycoside | 7.377 |
ND |
| Ephedrine | 18.413 |
ND |
| Proanthocyanin | 1.915 |
1.866 |
| SD: Saw dust, RB: Rice bran, RB/SD: Rice bran/saw dust substrate combination and ND: Not detected |
||
|
DISCUSSION
The need for the discovery and use of natural antidiabetic products has been necessitated by the exponential increase in diabetic cases and numerous side effects associated with the use of available hypoglycemic drugs. In the present study, the in vitro antidiabetic activity of aqueous extract of P. ostreatus grown on different substrates was evaluated. Results from this study showed that the mushroom extracts moderately inhibited α-amylase and α-glucosidase activities in vitro in a concentration-dependent manner with the highest percentage of inhibition occurring at the highest concentration of the mushroom samples. Although these enzymes play physiologically important roles in the digestion of carbohydrates, their over activity could contribute to persistent hyperglycemia, especially in conditions of impaired glucose clearance such as diabetes. The inhibitory effect of the P. ostreatus extracts indicates its potential to regulate postprandial hyperglycemia and suggests that it might be a potential candidate for improving diabetic conditions. This result was supported by the reports of Prabu and Kumuthakalavalli36, who stated a dose dependent inhibition of α-amylase and α-glucosidase activities in vitro by the aqueous extract of P. florida. Winska et al.37 also reported that, the aqueous extract of G. lucidum significantly decreased blood glucose levels in artificially induced diabetic rats.
Many disease conditions are associated with attenuated endogenous antioxidant defense systems leading to development and aggravation of complications associated with such diseases. In view of this, compounds with antioxidant capacity are always useful as adjuvant therapy in disease management.
DPPH, a stable free radical becomes a non-radical (DPPH-H) when it interacts with an antioxidant, donating an electron21 whereas, the ferric reducing antioxidant power (FRAP) method determines the ability of test substances to reduce Fe3+/ferric cyanide complex to its ferrous state38. The in vitro antioxidant analysis of the mushroom extracts showed moderate DPPH radical-scavenging activities and ferric reducing potentials of the extracts. This result correlates positively with the work of Anjana et al.21 in which they reported antioxidant activity of methanol extract of P. ostreatus with increase in extract concentration. Also, Stastny et al.39 reported significant antioxidant and anti-inflammatory activities of methanol extracts of P. ostreatus, P. florida and P. flabellatus in vitro.
As potent bio-accumulators, the chemical and nutritional profile of mushrooms is often a function of the type of substrate on which they are cultivated23,29. The gas chromatography analysis of the mushroom samples revealed the presence of a number of bioactive compounds, albeit in different concentrations. These compounds include epicatechin, naringin, tannin, kaempferol, rutin, amongst others. The presence of these compounds in these samples further highlights the health benefits associated with mushroom consumption. Epicatechin is a flavanol monomer with numerous health benefits including its positive effect on cardiovascular health and is also effective in the management of diabetes40. Rutin and kaempferol are also reported to exhibit a wide range of favorable biological effects including antioxidant, antihyperglycemic and neuroprotective effects8,41,42. The presence of these compounds in the mushroom homogenates is consistent with various reports16,21,43 in which they reported the presence of some of these compounds in mushroom samples. From the result, the RB/SD sample had higher concentrations of catechin, epicatechin, resveratrol, naringin and kaempferol compared to the SD sample while lunamarin and spartein were higher in the SD sample compared to the RB/SD sample. Rutin was below detectable limit in the RB/SD sample but present in the SD sample. These differences in biological compositions may be attributed to the difference in growth medium and this is consistent with the findings of Thongklang and Luangharn27 which indicated that the use of different substrates in the cultivation of P. ostreatus caused disparity in a lot of features of the mushroom. This was also supported by reports of Paul et al.44, that stated significant differences in proximate, mineral and vitamin contents of P. florida grown on different substrates. Mkhize et al.45 also reported significant differences in antioxidant activity of extracts from mushrooms cultivated on different substrates attributing this observation to differences in the chemical contents of the mushroom samples.
In the wake of the numerous side effects presented by different hypoglycemic agents, the findings of this study suggest that P. ostreatus can serve as a potential alternative in the management of diabetes and its complications.
The result of this study clearly demonstrates that P. ostreatus is a rich store of compounds which could be beneficial in disease conditions involving these carbohydrate-metabolizing enzymes. However, further studies would still be required to confirm if the in vitro observations translate to in vivo benefits as well.
CONCLUSION
From the result obtained, this study demonstrated the antidiabetic and antioxidant potentials of P. ostreatus aqueous extracts. It also highlighted that the substrates used in cultivation have a significant influence on the biological efficiency of mushrooms. The exact mechanism of enzyme inhibition as well as antioxidant activity was not provided by this study and thus should be a subject of further research. Bioactive compounds present in the P. ostreatus samples should be subjected to further studies and the relationship between mushroom content and substrates should also be investigated.
SIGNIFICANCE STATEMENT
Aqueous extract of P. ostreatus showed good inhibitory activity towards alpha-amylase and alpha-glucosidase suggesting that its potential in anti-diabetic activity could be explored further through in vivo study. Researchers, through the outcome of this study, can critically explore further the isolated bioactive compounds from P. ostreatus. This could enable an in-depth understanding of their medicinal effect and their future employment as antidiabetic agents.
REFERENCES
- Ceriello, A. and F. Prattichizzo, 2021. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol., 20.
- Jovanović, J.A., M. Mihailović, A. Uskoković, N. Grdović, S. Dinić and M. Vidaković, 2021. The effects of major mushroom bioactive compounds on mechanisms that control blood glucose level. J. Fungi, 7.
- Prabhakar, P.K., 2020. Hypoglycemic Potential of Mushroom and Their Metabolites. In: New and Future Developments in Microbial Biotechnology and Bioengineering: Recent Advances in Application of Fungi and Fungal Metabolites: Applications in Healthcare, Singh, J. and P. Gehlot (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 9780128210062, pp: 197-208.
- Sun, H., P. Saeedi, S. Karuranga, M. Pinkepank and K. Ogurtsova et al., 2022. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract., 183.
- Giacco, F., M. Brownlee and A.M. Schmidt, 2010. Oxidative stress and diabetic complications. Circ. Res., 107: 1058-1070.
- Sales, P.M., P.M. Souza, L.A. Simeoni, P.O. Magalhaes and D. Silveira, 2012. α-amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharmacy Pharm. Sci., 15: 141-183.
- Papoutsis, K., J. Zhang, M.C. Bowyer, N. Brunton, E.R. Gibney and J. Lyng, 2021. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem., 338.
- Peng, X., G. Zhang, Y. Liao and D. Gong, 2016. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem., 190: 207-215.
- Ni, M., X. Hu, D. Gong and G. Zhang, 2020. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocolloids, 105.
- Chatterjee, B. and T. Patel, 2016. Edible mushroom-A nutritious food improving human health. Int. J. Clin. Biomed. Res., 2: 34-37.
- Khursheed, R., S.K. Singh, S. Wadhwa, M. Gulati and A. Awasthi, 2020. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol., 164: 1194-1205.
- Zeb, M. and C.H. Lee, 2021. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules, 26.
- Nowakowski, P., R. Markiewicz-Żukowska, K. Gromkowska-Kępka, S.K. Naliwajko and J. Moskwa et al., 2021. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother., 133.
- Guissou, K.M.L., T.H. Coulidiati and M.N.T. Roland, 2020. Antioxidant activities and acetylcholine esterase inhibition of nine wild mushrooms from Burkina Faso. Int. J. Dev. Res., 10: 37432-37438.
- Muszyńska, B., A. Grzywacz-Kisielewska, K. Kała and J. Gdula-Argasińska, 2018. Anti-inflammatory properties of edible mushrooms: A review. Food Chem., 243: 373-381.
- Soares, A.A., A.B. de Sá-Nakanishi, A. Bracht, S.M.G. da Costa, E.A. Koehnlein, C.G.M. de Souza and R.M. Peralta, 2013. Hepatoprotective effects of mushrooms. Molecules, 18: 7609-7630.
- Shahzad, F., D. Anderson and M. Najafzadeh, 2020. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients, 12.
- Chowdhury, P. and S. Paul, 2020. The potential role of mushrooms in the prevention and treatment of diabetes: A review. J. Biol. Active Prod. Nat., 10: 429-454.
- Grimm, D. and H.A.B. Wösten, 2018. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol., 102: 7795-7803.
- Nongthombam, J., A. Kumar, Ladli, B. Manikanta, M. Madhushekhar and S. Patidar, 2021. A review on study of growth and cultivation of oyster mushroom. Plant Cell Biotechnol. Mol. Biol., 22: 55-65.
- Anjana, S.K.G., T.S.B. Balamurugan, V. Manivasagan and B.N.G. Ramesh, 2016. Phytochemical, antioxidant and antitumor activity of edible mushroom Pleurotus ostreatus. Int. J. Adv. Res. Biol. Sci., 3: 170-177.
- Venturella, G., V. Ferraro, F. Cirlincione and M.L. Gargano, 2021. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci., 22.
- Bellettini, M.B., F.A. Fiorda, H.A. Maieves, G.L. Teixeira and S. Ávila et al., 2019. Factors affecting mushroom Pleurotus spp. Saudi J. Bio. Sci., 26: 633-646.
- Koutrotsios, G., G. Danezis, C. Georgiou and G. Zervakis, 2020. Elemental content in Pleurotus ostreatus and Cyclocybe cylindracea mushrooms: Correlations with concentrations in cultivation substrates and effects on the production process. Molecules, 25.
- Raman, J., K.Y. Jang, Y.L. Oh, M. Oh, J.H. Im, H. Lakshmanan and V. Sabaratnam, 2021. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology, 49: 1-14
- Kumla, J., N. Suwannarach, K. Sujarit, W. Penkhrue and P. Kakumyan et al., 2020. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules, 25.
- Thongklang, N. and T. Luangharn, 2018. Testing agricultural wastes for the production of Pleurotus ostreatus. Mycosphere, 7: 766-772.
- Chukwurah, N.F., S.C. Eze, N.V. Chiejina, C.C. Onyeonagu and C.E.A. Okezie et al., 2013. Correlation of stipe length, pileus width and stipe girth of oyster mushroom (Pleurotus ostreatus) grown in different farm substrates. J. Agric. Biotechnol. Sustainable Dev., 5: 54-60.
- Aguchem, R.N., C.C. Chibuogwu, B.O. Okolo, U. Oyeagu and V.E. Etim et al., 2022. Nutrient and antinutrient composition of Pleurotus ostreatus grown on different substrates. Biol. Life Sci. Forum, 11.
- Narkhede, M.B., P.V. Ajimire, A.E. Wagh, M. Mohan and A.T. Shivashanmuga, 2011. In vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. Asian J. Plant Sci. Res., 1: 101-106.
- Gauthami, R., U.G. Vinitha, S.P. Anthony and M.S. Muthuraman, 2021. Cissampelous pairera mediated synthesis of silver nanoparticles and it’s in vitro antioxidant, antibacterial and antidiabetic activities. Mater. Today: Proceed., 47: 853-857.
- Sayah, K., I. Marmouzi, H.N. Mrabti, Y. Cherrah and M. El Abbes Faouzi, 2017. Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Res. Int., 2017.
- Adebiyi, O.E., F.O. Olayemi, T. Ning-Hua and Z. Guang-Zhi, 2017. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci., 6: 10-14.
- Alachaher, F.Z., S. Dali, N. Dida and D. Krouf, 2018. Comparison of phytochemical and antioxidant properties of extracts from flaxseed (Linum usitatissimum) using different solvents. Int. Food Res. J., 25: 75-82.
- Ojukwu, S.U., F.A. Onyegbule and C.E. Umeyor, 2021. GC-FID phytochemical analysis of selected antidiabetic herbal preparations sold in Onitsha Metropolis, Anambra State, Nigeria. J. Med. Plants Stud., 9: 60-67.
- Prabu, M. and R. Kumuthakalavalli, 2017. Antidiabetic potential of the oyster mushroom Pleurotus florida (Mont.) Singer. Int. J. Curr. Pharm. Sci., 9: 51-54.
- Wińska, K., W. Mączka, K. Gabryelska and M. Grabarczyk, 2019. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules, 24.
- El Omari, N., K. Sayah, S. Fettach, O. El Blidi and A. Bouyahya et al., 2019. Evaluation of in vitro antioxidant and antidiabetic activities of Aristolochia longa extracts. Evidence-Based Complementary Altern. Med., 2019.
- Stastny, J., P. Marsik, J. Tauchen, M. Bozik and A. Mascellani et al., 2022. Antioxidant and anti-inflammatory activity of five medicinal mushrooms of the genus Pleurotus. Antioxidants, 11.
- Dash, J.R., G. Pattnaik, G. Ghosh, G. Rath and B. Kar, 2023. Protective effect of epicatechin in diabetic-induced peripheral neuropathy: A review. J. Appl. Pharm. Sci., 13: 56-63.
- Ghorbani, A., 2017. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother., 96: 305-312.
- Guchu, B.M., A.K. Machocho, S.K. Mwihia and M.P. Ngugi, 2020. In vitro antioxidant activities of methanolic extracts of Caesalpinia volkensii Harms., Vernonia lasiopus O. Hoffm., and Acacia hockii De Wild. Evidence-Based Complementary Altern. Med., 2020.
- Kanjo, W.R., A.L. Njouonkou, A.K. Yongabi, T.F.P. Manfo, C. Tume and A.E. Nantia, 2022. In vitro screening of the anti-diabetic activity of six species of edible termite associated mushrooms (Termitomyces spp.) from the Western Highlands of Cameroon. Curr. Res. Environ. Appl. Mycol., 12: 125-135.
- Paul, R.K., D.K. Bhattacharjya, A.K.L. Kabir, M.H.O. Rashid and M.S. Rahaman et al., 2015. Effect of different saw dust substrates on the nutritional composition of oyster mushroom (Pleurotus florida) and its applications in human health. Dhaka Univ. J. Pharm. Sci., 14: 215-223.
- Mkhize, S.S., M.B.C. Simelane, I.N. Mongalo and O.J. Pooe, 2022. The effect of supplementing mushroom growing substrates on the bioactive compounds, antimicrobial activity, and antioxidant activity of Pleurotus ostreatus. Biochem. Res. Int., 2022.
How to Cite this paper?
APA-7 Style
Chukwurah,
C., Nnemolisa,
C.S., Chibuogwu,
C.C., Aguchem,
R.N., Ezeobi,
J.C., Ihionu,
A.N., Okagu,
I.U. (2023). In vitro Antidiabetic Studies of Aqueous Extract of Pleurotus ostreatus Grown on Different Substrates. Research Journal of Phytochemistry, 17(1), 37-47. https://doi.org/10.3923/rjp.2023.37.47
ACS Style
Chukwurah,
C.; Nnemolisa,
C.S.; Chibuogwu,
C.C.; Aguchem,
R.N.; Ezeobi,
J.C.; Ihionu,
A.N.; Okagu,
I.U. In vitro Antidiabetic Studies of Aqueous Extract of Pleurotus ostreatus Grown on Different Substrates. Res. J. Phytochem 2023, 17, 37-47. https://doi.org/10.3923/rjp.2023.37.47
AMA Style
Chukwurah
C, Nnemolisa
CS, Chibuogwu
CC, Aguchem
RN, Ezeobi
JC, Ihionu
AN, Okagu
IU. In vitro Antidiabetic Studies of Aqueous Extract of Pleurotus ostreatus Grown on Different Substrates. Research Journal of Phytochemistry. 2023; 17(1): 37-47. https://doi.org/10.3923/rjp.2023.37.47
Chicago/Turabian Style
Chukwurah, Chukwudumebi, Chukwubuikem Steven Nnemolisa, Christian Chiazor Chibuogwu, Rita Ngozi Aguchem, Jude Chinecherem Ezeobi, Anastasia Nwanneka Ihionu, and Innocent Uzochukwu Okagu.
2023. "In vitro Antidiabetic Studies of Aqueous Extract of Pleurotus ostreatus Grown on Different Substrates" Research Journal of Phytochemistry 17, no. 1: 37-47. https://doi.org/10.3923/rjp.2023.37.47

This work is licensed under a Creative Commons Attribution 4.0 International License.




