Ethnobotanical Survey on Sarcocephalus latifolius Smith and Antioxidant Activity of its Roots Extracts
| Received 18 Nov, 2022 |
Accepted 01 Mar, 2023 |
Published 06 May, 2023 |
Background and Objective: Nowadays, research on medicinal plants has intensified due to the diverse therapeutic potential of medicinal plants. The aim of this work is not only to go through the ethnobotanical knowledge of Sarcocephalus latifolius in Southern Benin but also to evaluate the antioxidant potential and toxicity of S. latifolius root extracts. Materials and Methods: Thus, an ethnobotanical survey was carried out in 06 markets located in Southern Benin. The antioxidant activity of S. latifolius root extracts was performed using the Ferric Reducing Antioxidant Power (FRAP) assay. To end, toxicity was performed on Artemia salina larvae following the dilution method. Results: The antioxidant activity revealed that the aqueous extract with IC50 of 0.299 mg mL‾1 has better antioxidant activity than the ethanolic extract (LC50 = 0.37 mg mL‾1). Larval toxicity shows that aqueous extract (LC50 = 0.528 mg mL‾1) and ethanolic extract (LC50 = 3.62 mg mL‾1) show no toxicity. Conclusion: The data of this study indicate that the extracts of S. latifolius present antioxidant and antimicrobial properties and do not present toxicity. This may justify its traditional use in the treatment of microbial infections.
INTRODUCTION
One of the options for local and natural solutions to solve health problems in Africa remains the use of plants. Indeed, medicinal plants occupy an important place in African pharmacopeia1. Research on medicinal plants has intensified due to the diverse therapeutic potential of these medicinal plants. The evaluation of plants in traditional medicine gives clues on how these plant parts can be used2. The use of plant extracts can be of great importance in therapeutic treatments3. Many plants have been used because of their antimicrobial characteristics that are due to their secondary metabolites contain. Sarcocephalus latifolius Smith (Rubiaceae) is used in many African countries by traditional medicine practitioners for the treatment of various ailments including bacterial diseases4.
In Africa, S. latifolius is widely used in traditional medicine to treat a variety of diseases including malaria, epilepsy, infectious diseases, dysentery, diarrhea, hernia, ascites, vomiting and colic4,5. In Benin, our previous study indicated antimicrobial activity on potential pathogenic microorganisms6. In addition, good in-vitro antioxidant, anti-inflammatory and anti-diabetic effects of this plant leaf and fruit extracts have also been reported7,8.
Antioxidants reported to significantly prevent the oxidation of oxidizable substrates at lower concentrations9. Thus, plants have long been a source of antioxidants and the major part of medicinal plants’ importance has their excellent antioxidant potential10.
Among the potential plant, S. latifolius has been identified and used due to its medicinal properties regarding gastric disorders and foodborne diseases11. These studies support the fact that traditional medicine still has unexplored potential. Meanwhile, the main problem with using traditional treatments is the lack of scientific knowledge regarding efficacy, indications, properties and quality control. After the study on the physicochemical composition and antimicrobial activity of S. latifolius, it seems important to investigate the antioxidant activity and toxicity of its extracts6. Thus, the present study aimed to investigate the ethnobotanical knowledge and determine the antioxidant potential and toxicity of S. latifolius root extracts.
MATERIALS AND METHODS
Ethnobotanical survey on the medicinal use of Sarcocephalus latifolius: In this study, an ethnobotanical survey was carried out on Sarcocephalus latifolius and its use in disease treatment in Southern Benin. This survey took place in four localities namely Abomey-Calavi (3 markets), Cotonou (2 markets) and Ouidah. It was carried out on 33 people and was carried out among phytotherapists, herbalists and individuals living in contact with medicinal plants. A personal survey was used according to the work of Roko et al.12. The data collected during the study, from April, 2022 to June, 2022, were related to the socio-demographic characteristics of phytotherapists and herbalists and their medicinal use of S. latifolius.
Samples collection and pulverization: The roots of Sarcocephalus latifolius were collected from the surveyed area and certified (N°YH687/HNB) at the National Herbarium of Benin. Once certified, collected samples were cleaned and dried at room temperature (22±2°C) for about 14 days. After drying, root samples were powdered (Retsch mill SM 2000/1430/Upm/Smf) and stored until used for the antioxidant activity and toxicity.
Samples analysis
Obtaining the extracts: Ethanolic and aqueous extracts obtained according to a previously developed method13 were used in this study. The choice of these types of extracts is based on the way the plant is traditionally used. For the aqueous extracts, 50 g of obtained powder was macerated in 500 mL of distilled water for 72 hrs under continuous stirring. The obtained homogenate was successively filtered three times on absorbent cotton and once on Whatman paper. This filtrate was then dried in an oven at 50°C and the powder obtained constitutes the total aqueous extract. Concerning the ethanolic, 50 g of powder was macerated in 500 mL of 96% ethanol for 72 hrs under continuous stirring. The mixture was then filtered three times on absorbent cotton and once on Whatman No. 1 to obtain a solids-free solution. The filtrate was concentrated in a rotary evaporator at 50°C and stored at 2-4°C.
Evaluation of the antioxidant activity: The reducing power of the extracts was determined by the Ferric Reducing Antioxidant Power (FRAP) method according to the protocol described by Dieng et al.14. Thus, 0.5 mL of 25% DMSO was distributed in all tubes (2 to 7) and then 0.5 mL of the extract at 5 mg mL–1 was introduced in tubes 1 and 2. Then a series of successive ½ dilutions were performed in all the other tubes. As 0.5 mL of sample at different concentrations was mixed with 1 mL of phosphate buffer (0.2 M, pH = 6.6) and 1 mL of 1% potassium hexacyanoferrate [K3Fe(CN)6]. After incubating the mixture at 50°C for 30 min, 1 mL of 10% trichloroacetic acid was added to stop the reaction, then the tubes were centrifuged at 3000 rpm for 10 min. Then, 1 mL of the supernatant from each tube was mixed with 0.2 mL of 0.1% FeCl3 solution and allowed to stand in the dark for 30 min before measuring the optical densities at 700 nm. The antioxidant activity related to the reducing power of the extracts is expressed as Reducing Power (RP) using the following formula:
| OD | = | Optical density |
The IC50s (Concentration that inhibits the 50% of DPPH or reduces the 50% of Fe3+) were determined by the probit method13-14.
Larval toxicity test evaluation: The evaluation of larval toxicity was highlighted by a slightly modified method by Kawsar et al.15. Thus, 10 mg of Artemia salina eggs were placed under continuous agitation in 1 L of seawater for 72 hrs. After the larvae hatched, a stock solution of the test extract at 20 mg mL–1 was prepared. From 1 mL of distilled water in each tube (except the first tube) and then 1 mL of the test extract, successive dilutions to 1/2 were made. Then, 16 larvae of Artemia salina were introduced in each of the 10 dilution tubes so as to have 2 mL after the addition of the 16 larvae. After 24 hrs of incubation, live, moribund and dead larvae were counted for LC50 determination. To assess the degree of toxicity of the extracts, the table of correspondence between LC50 and toxicity previously established13 is used.
Statistical analysis: Acquired data were analyses using GraphPad Prism 8 software. For each extract, the lethal concentration that causes 50% larval death (LC50) was calculated with a 95% confidence interval by linear regression analysis and also using the Probit analysis method following. A regression line equation, obtained from the larval mortality curve, is used to calculate the concentration (LC50) corresponding to the death of half the larvae.
RESULTS
Ethnobotanical survey on the medicinal use of Sarcocephalus latifolius
Socio-demographic characteristics: The socio-demographic parameters of the respondents. The analysis of this table showed that of the 33 respondents whom all spoke Fon, 24.24% were male and 75.75% were female. The age range varies from 20 to 80 years with an average age of 49.65±12.82. Note that 12.12% do not know their ages. Regarding the level of education, the majority of the people are illiterate (79%) and the rest are distributed according to primary level education (9%), secondary education (9%) and university education (3%). Also, the professional experience varies from 5 to 35 years with an average of 14.69±7. It should be noted that 21.21% did not know the duration of their professional experience as shown in Table 1.
Pharmacological and medicinal use of Sarcocephalus latifolius: The pharmacological and medicinal use of S. latifolius. It appears from its analysis that the roots are used to treat various types of diseases. Thus, about 19 diseases have been identified as a result of the traditional use of the plant, these include malaria, stomach ache, headache, painful menstruation, sepsis, thrombosis, hemorrhoid, ulcer, cold, cough, lower abdominal problems, hypogalactia, jaundice, vaginal infection, diabetes, infection, fatigue, erectile dysfunction and chronic cough. The treatment is based on one of the following methods of preparation, depending on the pathology to be treated. One distinguishes the simple decoction, the decoction (root+lemon), alcoholature, or the decoction (root+kpédjrékoun+atinkingbata+ahowé+sassalinkoun). The dosage was based on the administration of a bamboo glass morning noon evening or morning evening as presented in Table 2.
| Table 1: | Socio-demographic parameters of the respondents | |||
| Socio-demographic parameter | Number |
Percentage (%) |
| Sex | ||
| M | 8 |
24.24 |
| F | 25 |
75.75 |
| Age | ||
| 20-40 years | 5 |
15.15 |
| 40-60 years | 17 |
51.51 |
| 60-80 years | 7 |
21.21 |
| Level of study | ||
| Not know | 4 |
12.12 |
| Primary | 3 |
9 |
| Secondary | 3 |
9 |
| University | 1 |
3 |
| Illiterate | 26 |
79 |
| Professional experience | ||
| 5, 15 | 15 |
45.45 |
| 15, 25 | 8 |
24.24 |
| 25, 35 | 3 |
9.09 |
| Not know | 7 |
21.21 |
| Table 2: | Pharmacological and medicinal use of the root of Sarcocephalus latifolius | |||
| Medicinal use | |||
| Part used | Pathology | Method of preparation | Posology |
| Root | Malaria, stomach ache, headache, painful | Decoction | 1 bamboo glass morning noon |
| menstruation, sepsis, thrombosis, hemorrhoid, | evening | ||
| ulcer, cold, cough, lower abdominal problems, | |||
| hypogalactia, jaundice, vaginal infection, diabetes | |||
| Malaria, infection, stomach ache, headache, | Decoction (Root+lemon) | 1 bamboo glass morning noon | |
| painful menstruation, thrombosis and vaginal infection |
evening | ||
| Malaria, stomach ache, Fatigue, erectile | Alcoholature | ||
| dysfunction, painful menstruation | |||
| Chronic cough | Decoction (root+kpédjrékoun | ||
| +atinkingbata+ahowé | |||
| +sassalinkoun) | |||
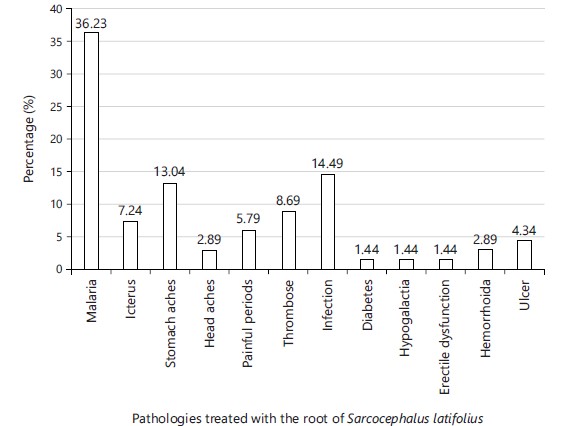
The pathologies treated with the root of S. latifolius according to the respondents. From the analysis of this fig, the most treated pathologies by the roots of Sarcocephalus latifolius are malaria (36.23%), infections (14.49%), stomach ache (13.04%), thrombosis (8.69%), icterus (7.24%), painful periods (5.79%). Some diseases are also treated with a relatively low percentage, these are ulcers (4.34%), hemorrhoids (2.89%), headaches (2.89%) and others with negligible percentages: diabetes (1.44%), hypogalactia (1.44%) and weak erection (1.44%) as shown in Fig. 1.
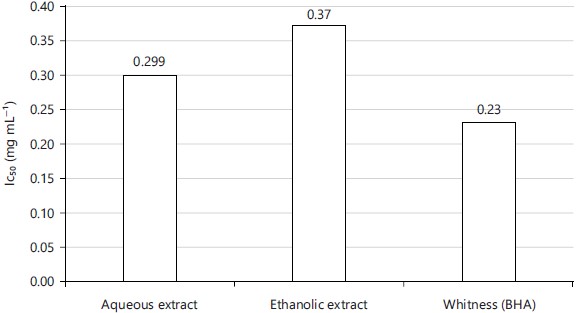
Antioxidant power of extracts: The Inhibitory Half Concentration (IC50) of the two root extracts of S. latifolius and of the control. It emerges from his analysis that the aqueous and ethanolic extracts respectively have IC50s of 0.299 and 0.37 mg mL–1, whereas the IC50 of the control is 0.23 mg mL–1. It should be remembered that the lower the IC50 value, the greater the antioxidant activity of a compound as shown in Fig. 2.
|
|
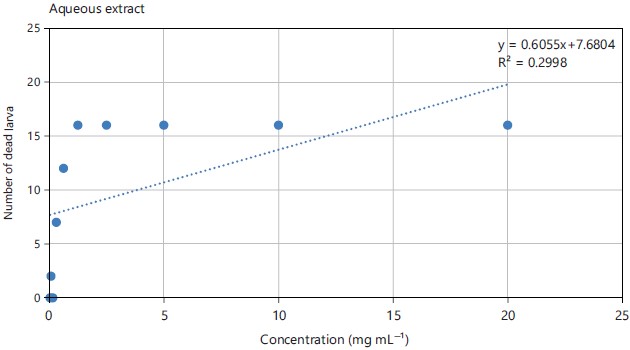
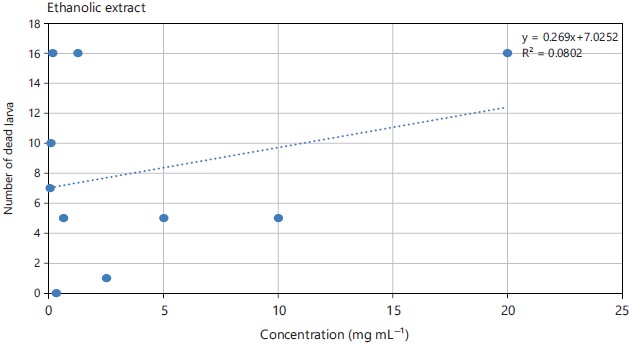
Larval toxicity: From the number of dead larvae recorded at the end of the test, the curves variation of the sensitivity of the larval cells were drawn according to the different concentrations of the extracts. Figure 3 and 4 showed the regression curve expressing the number of dead larvae as a function of the concentration of the aqueous and ethanolic extracts root of Sarcocephalus latifolius. From the analysis of these graphs, it can be seen that the correlation coefficient R2 is less than 0.8 for the two extracts, i.e. 0.2998 for the aqueous extract and 0.0802 for the ethanolic extract. In addition, the larvae are more sensitive to the aqueous extract than to the ethanolic extract. From the sensitivity curve below, the LC50 of the aqueous extract was equal to 0.528 mg mL–1 and that of the ethanolic extract was 3.62 mg mL–1 (Table 3).
|
|
| Table 3: | Correspondence between LC50 and cytotoxicity | |||
| LC50 | Cytotoxicity |
| LC50>100 μg mL–1 | -(no toxicity) |
| 100 μg mL–1>LC50 >50 μg mL–1 | +(low toxicity) |
| 50 μg mL–1>LC50 >10 μg mL–1 | ++(average toxicity) |
| LC50 <10 μg mL–1 | +++(high toxicity) |
DISCUSSION
The ethnobotanical study carried out showed that of the 33 people surveyed, 25 were female and 8 were male, these results were different from those reported in Mali16. The author reported that of the 25 people surveyed, 23 were male and 2 were female. This may be due to the area of the survey and the fact that the female gender is still not a holder of traditional knowledge. During this survey, it was noted that the root of S. latifolius is used in the treatment of 19 diseases and infections, these results were contrary to those of Haidara et al.16 who found that the population uses the leaves, trunk bark, roots and even fruits in the treatment of 13 diseases and infections. This difference may be due to the lack of knowledge of the species, especially the different organs used in the treatment of diseases.
The aqueous and ethanolic extracts have an inhibitory half-concentration (IC50) of 0.299 and 0.37 mg mL–1, respectively. The lower the IC50, the greater the antioxidant activity the compound has. Among the extracts tested, the lowest IC50 being 0.299 mg mL–1 for the aqueous extract, it can therefore be deduced that the aqueous extract has better antioxidant activity better than the ethanolic extract even if the two extracts have the capacity to reduce ferric ions as shown in Fig. 4. These results were contrary to those found by Iheagwam et al.7, who reported that the ethanolic extract (1.19±0.11 mg mL–1) of the leaves of S. latifolius has a strong antioxidant power unlike the aqueous extract (2.64±0.48 mg mL–1). This difference in results may be due to the part of the plant used. The results obtained in this study suggested that the aqueous extract concentrates the most chemical constituent and has the ability to donate hydrogen to a free radical17. The presence of phtytocompounds, in this case, the flavonoids observed in this work, would justify the antioxidant activity of the aqueous extract which would be due to its richness in phenolic compounds. Several studies have demonstrated the antioxidant power of phenolic compounds and particularly flavonoids17.
The analysis of larval toxicity curves shows that the aqueous (LC50 = 0.528 mg mL–1) and ethanolic (LC50 = 3.62 mg mL–1) extracts showed no toxicity according to the table established13. The root of Sarcocephalus latifolius therefore, does not present any toxicity whatever the solvent used. These results were consistent with those reported by Onzo et al.18 (5.183 mg mL–1) having reported the non-toxicity of extracts from the leaves of S. latifolius. The non-toxicity of the root of S. latifolius corroborates the results of the phytochemical screening which showed the absence of cardiotonic glycosides and cyanogenic derivatives which are generally toxic compounds19,20.
The roots of Sarcocephalus latifolius are wildly used in Southern Benin in the treatment of server diseases. Its ethanolic and aqueous extracts are not toxic and show good antioxidant activity.
CONCLUSION
This work confirmed or invalidate the practice of medicinal plants and represents a step forward in the improvement of traditional medicine in general and in particular in the rational exploitation of Sarcocephalus latifolius. The antioxidant activity revealed that of the two extracts tested, the aqueous extract has better antioxidant power than the ethanolic extract. The toxicity carried out on Artemia salina larvae revealed that the aqueous and ethanolic extracts show no toxicity. Because of these results, the use of the root of S. latifolius in traditional medicine to treat pulmonary infections in general and pneumococcal diseases, in particular, is justified. However, additional toxicity studies are needed to demonstrate the safety of S. latifolius root extracts.
SIGNIFICANCE STATEMENT
This study elucidated the ethnopharmacology and traditional uses of Sarcocephalus latifolius in Southern Benin. In addition, the toxicity and antioxidant capability of the root extracts of this plant are well known. Thus, this study will help populations to better use the S. latifolius base traditional medicines.
REFERENCES
- Mahomoodally, M.F., 2013. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evidence-Based Complementary Altern. Med., 2013: 617459.
- Haudecoeur, R., M. Peuchmaur, B. Pérès, M. Rome, G.S. Taïwe, A. Boumendjel and B. Boucherle, 2018. Traditional uses, phytochemistry and pharmacological properties of African Nauclea species: A review. J. Ethnopharmacol., 212: 106-136.
- Prabuseenivasan, S., M. Jayakumar and S. Ignacimuthu, 2006. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med., 6: 39.
- Boucherle, B., R. Haudecoeur, E.F. Queiroz, M. de Waard, J.L. Wolfender, R.J. Robins and A. Boumendjel, 2016. Nauclea latifolia: Biological activity and alkaloid phytochemistry of a West African tree. Nat. Prod. Rep., 33: 1034-1043.
- Okwu, D.E. and N.F. Uchenna, 2009. Exotic multifaceted medicinal plants of drugs and pharmaceutical industries. Afr. J. Biotechnol., 8: 7271-7282.
- Chabi-Sika, K., H. Sina, B. Boya, I. Mama-Sirou and L. Kpangon et al., 2022. Phytochemical screening and antimicrobial activity of Sarcocephalus latifolius smith roots extracts. Biotechnol. J. Int., 26: 54-62.
- Iheagwam, F.N., E.N. Israel, K.O. Kayode, O.C. DeCampos, O.O. Ogunlana and S.N. Chinedu, 2020. Nauclea latifolia Sm. leaf extracts extenuates free radicals, inflammation, and diabetes-linked enzymes. Oxid. Med. Cell. Longevity, 2020: 5612486.
- Ayeleso, A.O., O.O. Oguntibeju and N.L. Brooks, 2014. In vitro study on the antioxidant potentials of the leaves and fruits of Nauclea latifolia. Sci. World J., 2014: 437081.
- Halliwell, B., 2007. Biochemistry of oxidative stress. Biochem. Soc. Trans., 35: 1147-1150.
- Krishnaiah, D., R. Sarbatly and R. Nithyanandam, 2011. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process., 89: 217-233.
- Kaboré, S.A., M. Hien, D. Ouédraogo, T.R.E. Diallo, K. Hahn and H.B. Nacro, 2014. Use of ecosystem services of Sarcocephalus latifolius (Sm.) E.A.Bruce and induced effect of human pressure on the species in the Southwestern Region of Burkina Faso. Ethnobotany Res. Appl., 12: 561-570.
- Roko, O.G., V. Dougnon, A. Hounkpatin, J.R. Klotoé and L. Baba-Moussa, 2019. Anti-inflammatory, analgesic and antipyretic properties of ethanolic extracts of three plants of Beninese’s pharmacopoeia: Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia. Asian J. Biol., 8: 54667.
- Chabi-Sika, K., H. Sina, B. Boya, F. Bade and T. Hounnou et al., 2021. Richardia brasiliensis collected in Southern-Benin: Phytochemical screening, antimicrobial activity and toxicity. Asian J. Biol., 13: 22-33.
- Dieng, S.I.M., A.D. Fall, K. Diatta-Badji, A. Sarr and M. Sene et al., 2017. Evaluation of the antioxidant activity of hydro-ethanolic extracts of the leaves and barks of Piliostigma thonningii Schumach. Int. J. Biol. Chem. Sci., 11: 768-776.
- Kawsar, S.M.A., E. Huq and N. Nahar, 2008. Cytotoxicity assessment of the aerial parts of Macrotyloma uniflorum Linn. Int. J. Pharmacol., 4: 297-300.
- Haidara, M., G. Bourdy, N. de Tommasi, A. Braca, K. Traore, S. Giani and R. Sanogo, 2016. Medicinal plants used in Mali for the treatment of malaria and liver diseases. Nat. Prod. Commun., 11: 339-352.
- Georges, K.A., A.A.R. Constant, E. Lynda, E. Tchirioua and K.M. Witabouna, 2018. Antioxidant activity of some plants used in the region of Tiassalé (Côte d'Ivoire) in the maintenance of skin health. Eur. Sci. J., 14: 338-352.
- Onzo, C.F., A. Adjatin, F. Assogba, H.A. Ndtoungou and H.W. Djengue et al., 2016. Domestication potential of plant leaf species used as food packaging in Benin. Int. J. Innovation Appl. Stud., 18: 539-550.
- Gandonou, D.C., H. Ahissou, J.M. Tokoudagba and C. Dansou, 2017. Ethnobotanical, phytochemical and toxicity analysis of a beninese antihypertensive plant: Lippia multiflora. Int. J. Biol. Chem. Sci., 11: 1816-1828.
- Samba, N., R. Aitfella-Lahlou, M. Nelo, L. Silva, R. Coca, P. Rocha and J.M.L. Rodilla, 2021. Chemical composition and antibacterial activity of Lippia multiflora Moldenke essential oil from different regions of Angola. Molecules, 26: 155.
How to Cite this paper?
APA-7 Style
Chabi-Sika,
K., Mama-Sirou,
I., Sina,
H., Boya,
B., Roko,
G., Kpangon,
L., Salami,
H.A., Amogou,
O., Dah-Nouvlessounon,
D., Tohoyessou,
M.G., Baba-Moussa,
L. (2023). Ethnobotanical Survey on Sarcocephalus latifolius Smith and Antioxidant Activity of its Roots Extracts. Research Journal of Phytochemistry, 17(1), 11-18. https://doi.org/10.3923/rjphyto.2023.11.18
ACS Style
Chabi-Sika,
K.; Mama-Sirou,
I.; Sina,
H.; Boya,
B.; Roko,
G.; Kpangon,
L.; Salami,
H.A.; Amogou,
O.; Dah-Nouvlessounon,
D.; Tohoyessou,
M.G.; Baba-Moussa,
L. Ethnobotanical Survey on Sarcocephalus latifolius Smith and Antioxidant Activity of its Roots Extracts. Res. J. Phytochem 2023, 17, 11-18. https://doi.org/10.3923/rjphyto.2023.11.18
AMA Style
Chabi-Sika
K, Mama-Sirou
I, Sina
H, Boya
B, Roko
G, Kpangon
L, Salami
HA, Amogou
O, Dah-Nouvlessounon
D, Tohoyessou
MG, Baba-Moussa
L. Ethnobotanical Survey on Sarcocephalus latifolius Smith and Antioxidant Activity of its Roots Extracts. Research Journal of Phytochemistry. 2023; 17(1): 11-18. https://doi.org/10.3923/rjphyto.2023.11.18
Chicago/Turabian Style
Chabi-Sika, Kamirou, Ibrahima Mama-Sirou, Haziz Sina, Bawa Boya, Gautier Roko, Lucas Kpangon, Hafiz Adio Salami, Olaréwadjou Amogou, Durand Dah-Nouvlessounon, Majoie Géroxie Tohoyessou, and Lamine Baba-Moussa.
2023. "Ethnobotanical Survey on Sarcocephalus latifolius Smith and Antioxidant Activity of its Roots Extracts" Research Journal of Phytochemistry 17, no. 1: 11-18. https://doi.org/10.3923/rjphyto.2023.11.18

This work is licensed under a Creative Commons Attribution 4.0 International License.




